DCT Infographic
A summary graphic on the September 2024 Final DCT Guidance
A Summary Graphic on the FDA DCT Guidance
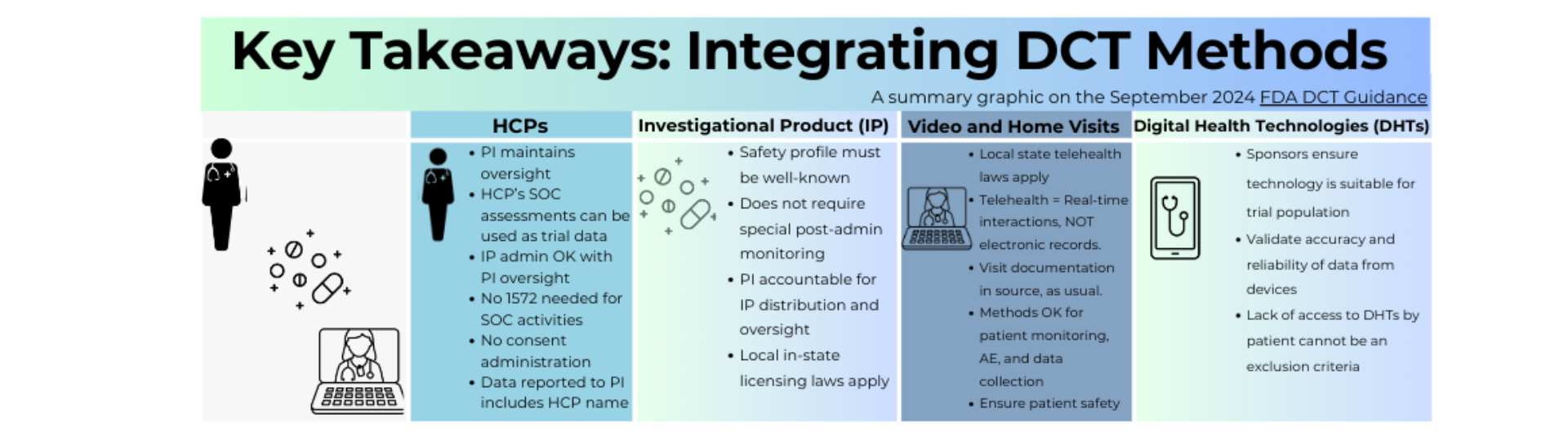
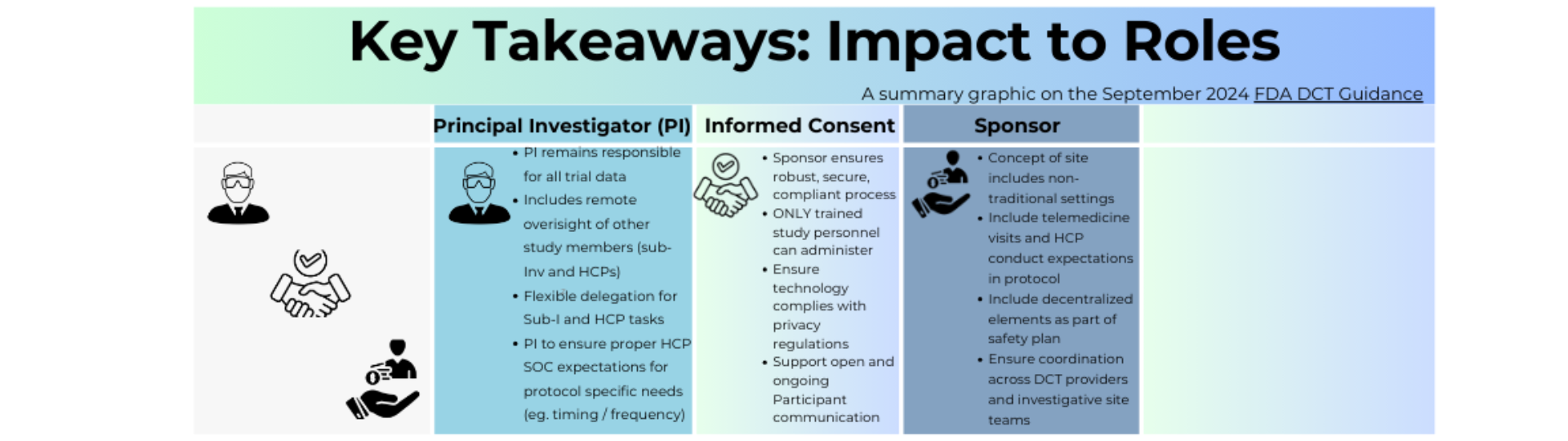
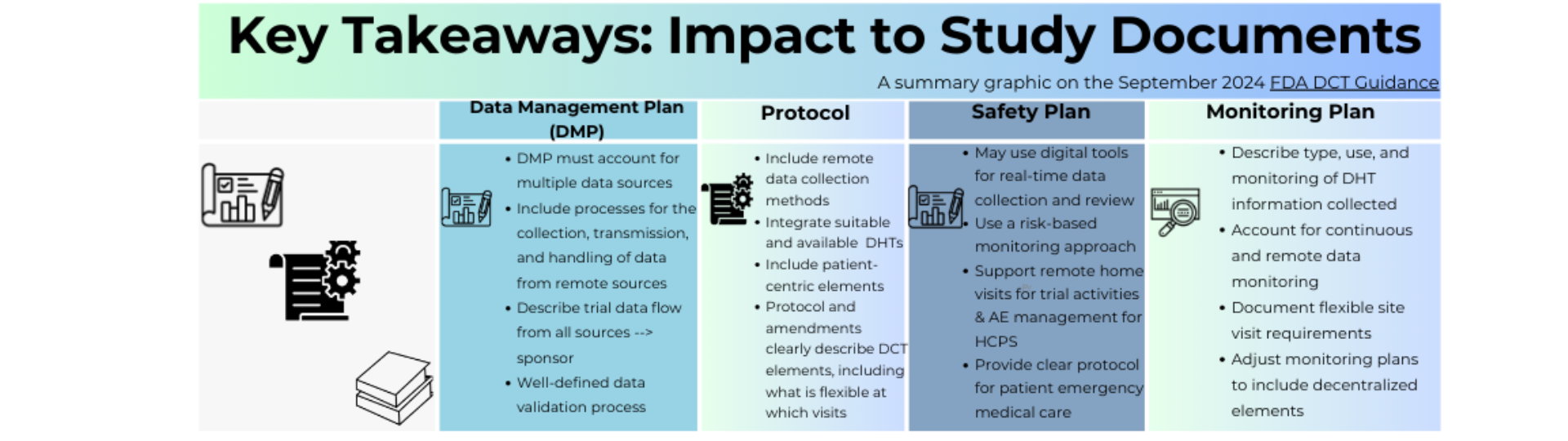
The Final DCT Guidance from the FDA, released in September 2024, provided recommendations on how to conduct clinical trials using DCT elements while maintaining regulatory standards. This Infographic highlights key aspects of the guidance on implementing DCTs elements focusing on regulations and best practices for conducting clinical trials that use these approaches. This aim to provide a simplistic overview of the guidance's key themes, ensuring easy understanding for our community as we work to drive DCT adoption.