1572 CoLab
1572 and Regulatory Document Recommendations for DCTs

1572 and Regulatory Document
Recommendations for DCTs:
Scope
Recommendations on if/how to best include DCT-specific roles and needs (eg. local labs, local imaging, local healthcare providers) using the 1572 or other forms

1572 and Regulatory Document
Recommendations for DCTs:
Deliverables
Create recommendations for 1572 / regulatory form completion to document
- Conduct of decentralized trial assessments
- Oversight responsibility for decentralized trial assessments
- Use of virtual sites / metasites, mobile nursing services, retail pharmacy, local community physicians, local imaging centers, local labs, and appropriate documentation guidance
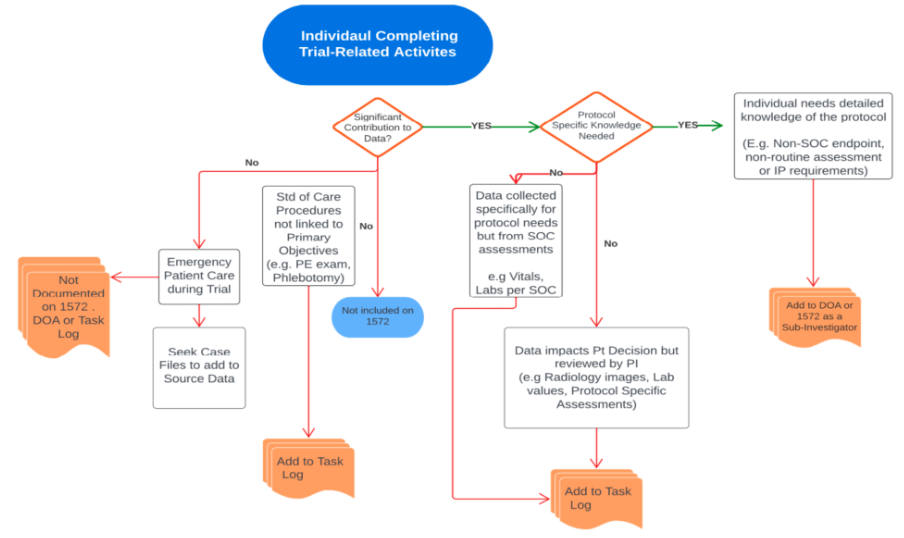
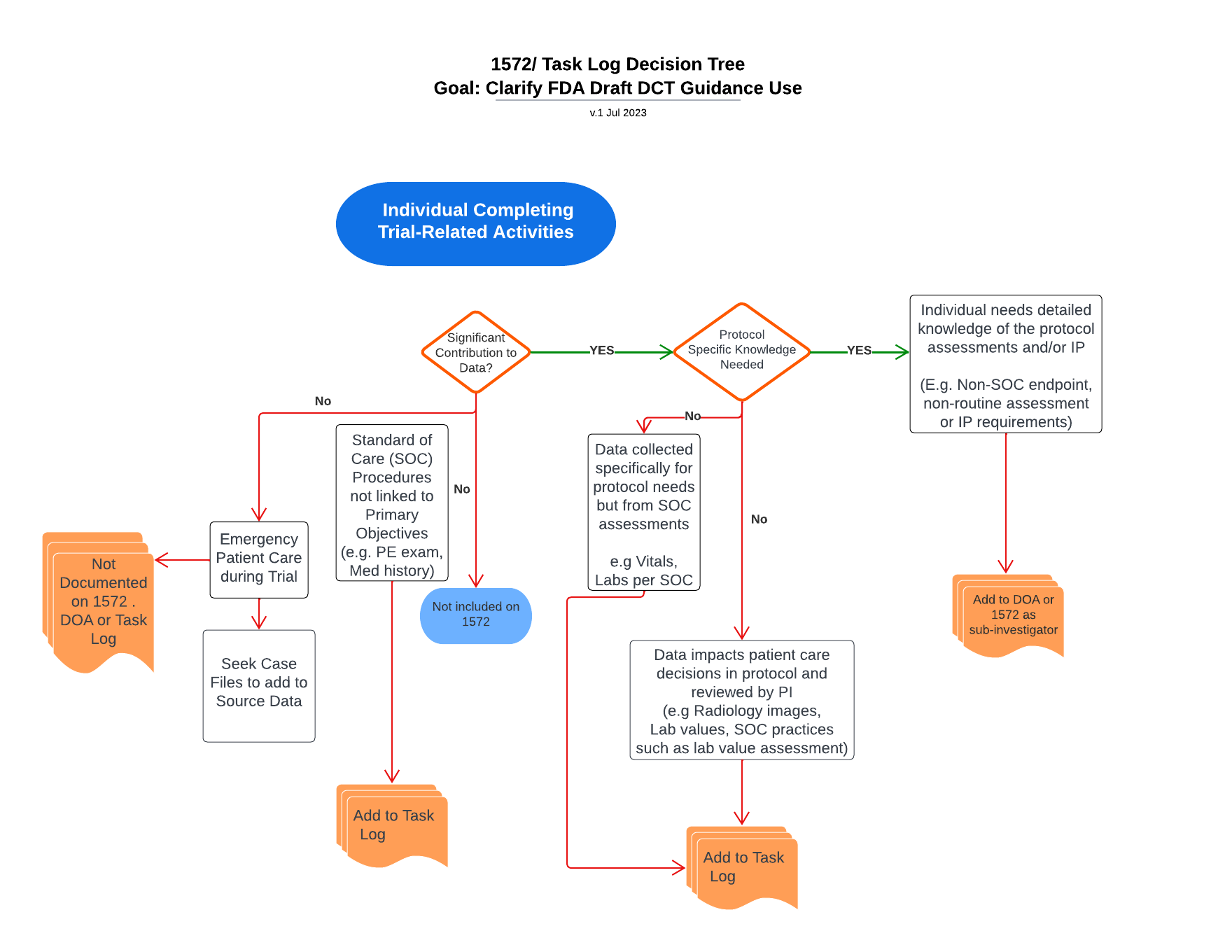
Decision elements to determine appropriate documentation of delegated trial-related activities
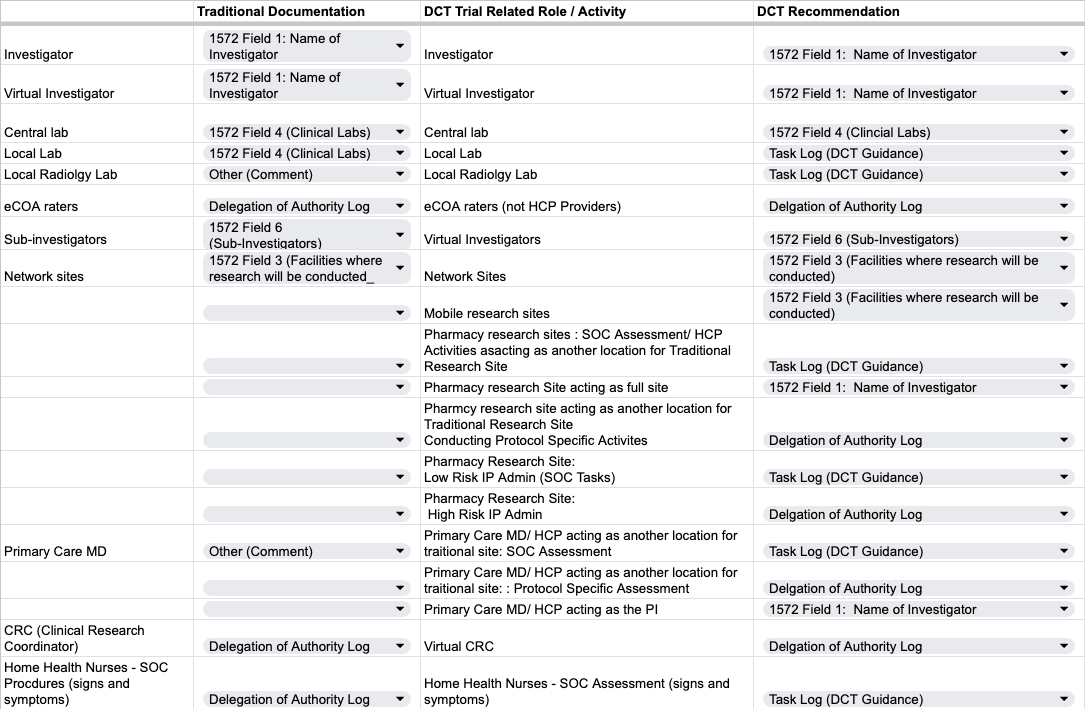

Resource Table - Traditional & DCT Roles and Documentation Recommendations
Use this to as a guidance on how completing the forms is similar and different between DCTs and traditional trials

Decision elements to determine appropriate documentation of delegated trial-related activities
Use this tool to help consider which roles are documented in which forms based on the activities they are performing.
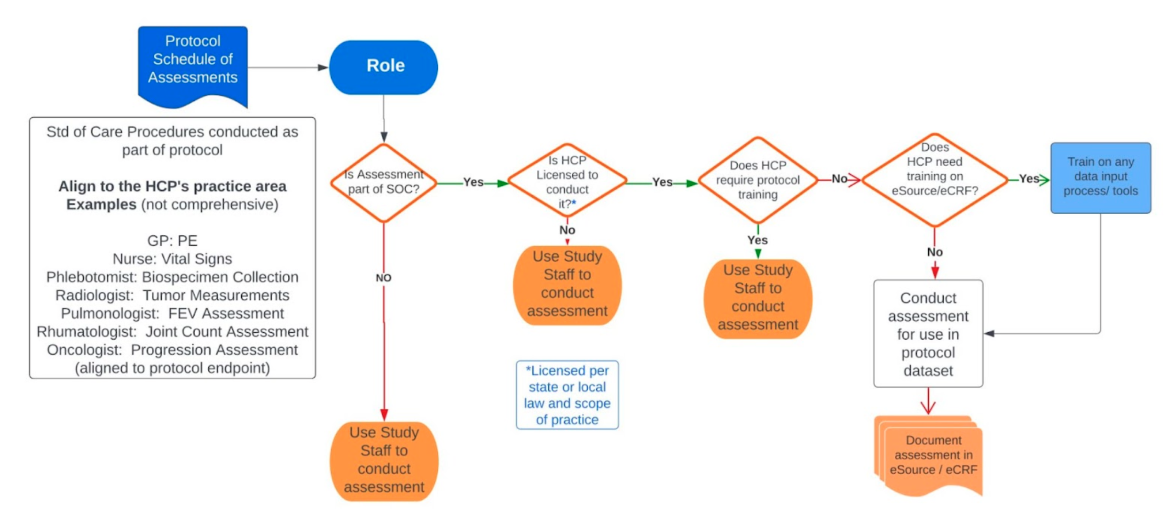

Decision Elements regrading Standard of Care Practice
Use this tool to help decide which procedures could be considered standard of care and what questions to ask.
Scenarios for PI Oversight and Delegation of Trial-Related Activities
The Co-Lab Team created two trial scenarios and examples on the documentation for each. We've created one scenario for an RSV trial and one for a rare disease trial. Each scenario includes:
- A patient journey map
- A simple explanation of how one site will operationalize the trial.
- An annotated 1572 form and the DOA / Task log that aligns to that site model
