Site Input to Protocol Planning How-To Guide
How to Use the Site Input Framework
A practical, two-touchpoint process for improving trial design, feasibility, and adoption of decentralized trial methods.

Why Site Input Matters
Sites see what protocols miss. Gathering their input early, and using it intentionally, reduces burden, prevents avoidable delays, and strengthens adoption of decentralized trial elements.
Where Site Input fits in the Planning Process
Before Protocol Finalization
- Understand patient burden
- Understand site burden
- Evaluate feasibility of DCT elements
- Make design changes while you still can
Before Site Selection
- Access site capabilities and experience
- Identify logistical and workflow barriers
- Align expectations between site sponsors, CRO, and vendors
Sponsors can use one or both stages depending on study needs.
The Four-Step Process

Choose Method
- Surveys or short micro-questionnaires
- Interviews or focus groups with coordinators and investigators
- Site advisory boards
- Protocol or technology simulations
- Feasibility or SIV discussions, if an amendment is planned

Communication
- Explain the purpose of the input and how it will be used
- Clarify which roles you want to resond (PI, CRC, Site Director, etc)
- Confirm timelines, effort required, and how results will be shared back
- Reassure sites about confidentiality and how answers will (and will not) be used

Use the Question Bank
- Configure your Schedule of Assessments using the Icons Library. That helps you and the site members clearly understand what's being proposed.
- Filter by protocol area, indication, timepoint, and respondent role
- Use Core questions when time is tight
- Add High-Value questions for feasibility and workflor detail
- Add Deep-Dive questions when piloting new or complex DCT elements

Act on Feedback
Translate site input into clear actions in two domains:
Design adjustments (when changes are still possible)
- Simplify visit schedules or consolidate touchpoints
- Add, remove, or modify remote options
- Adapt procedures to reduce unnecessary burden
- Align DCT elements with patient preference and site capability
Operational alignment (when the protocol is fixed)
- Select or configure technology that sites can realistically use
- Tailor training materials to site-reported gaps
- Clarify responsibilities across sponsor, CRO, vendors, and sites
- Align budgets with actual site workload
- Share key insights with vendors to set them up for success
Close the Loop
Share back what you heard, what changed, and what sites can expect going forward
Before Protocol Finalization
- Summarize major themes from site input
- Highlight protocol changes made to reduce burden or improve feasibility
- Note any DCT elements that directly affect primary/secondary endpoints
Before Site Selection
- Clarify expectations for how sites will interact with DCT vendors and tools
- Outline workflows, help-desk contacts, and communication plans
- Confirm how site activities related to DCT elements are reflected in budgets
Share with Stakeholders
- Sites and investigators
- Sponsor study teams
- CROs and DCT vendors
- Other operational partners
This step turns a one-time consultation into an ongoing partnership and increases the likelihood that sites will engage in future input requests
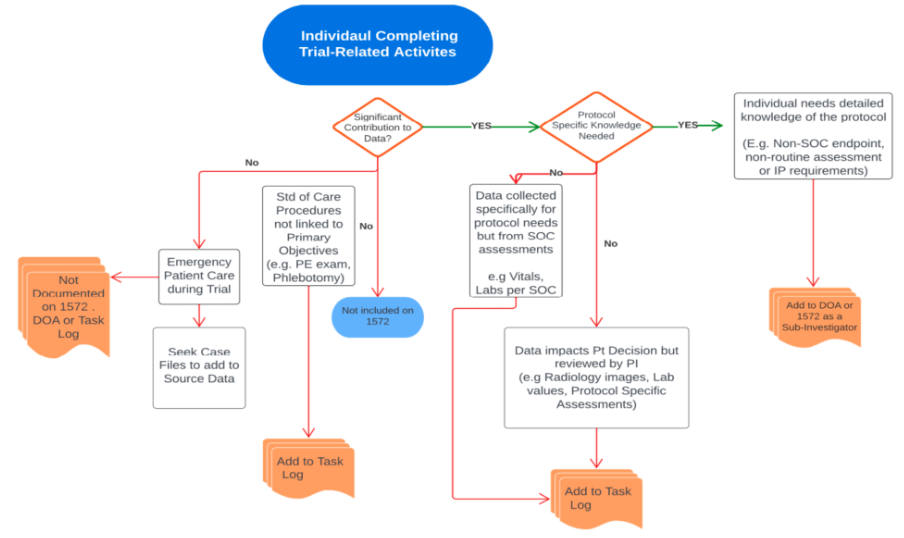
Decision elements to determine appropriate documentation of delegated trial-related activities