Site Input to Protocol Planning CoLab
A practical framework for sponsors to include site input when using DCT elements

The How-To Guide for Site Input
This page links to three tools designed for sponsors to get site input to enable early, practical decisions about utilizing decentralized methods:
- Playbook: A streamlined process for collecting and using site input.
- Question Bank: Focused questions aligned with key decision points.
- Standard DCT Icons: A visual language to clarify who does what, when.

Why this matters:
DCT elements often reach sites after the protocol is locked—too late to adjust schedules, workflows, or support. That’s when burden increases and adoption drops.
This CoLab provides a simple, repeatable process to gather site input early enough to influence protocol decisions without slowing development timelines.

Who created this?
DTRA chartered a team to develop a collaborative framework in partnership with:
- Transcelerate Biopharma
- Association of Clinical Research Professionals (ACRP)
- Society for Clinical Research Sites (SCRS)
Additional contributions were provided by global sponsors, CROs, clinical research sites, and technology partners.

Co-Created with Sites
The CoLab teams led the core design and analysis. The first step was to understand the problem and pressure test ideas through one one one interviews with different site types. Solutions were developed based on that input, and tested with site representatives, including in a workshop held at SCRS Global Summit.
- 2023: Prioritized the core problem statement with sites.
- 2024: Tested early concepts to confirm what sites need to execute DCT elements reliably
- 2025: Validated and refined this CoLab's specific outputs with a broader set of sites and geographies.
These workshops sharpened the tools but the majority of the content was build by the initaitive teams.
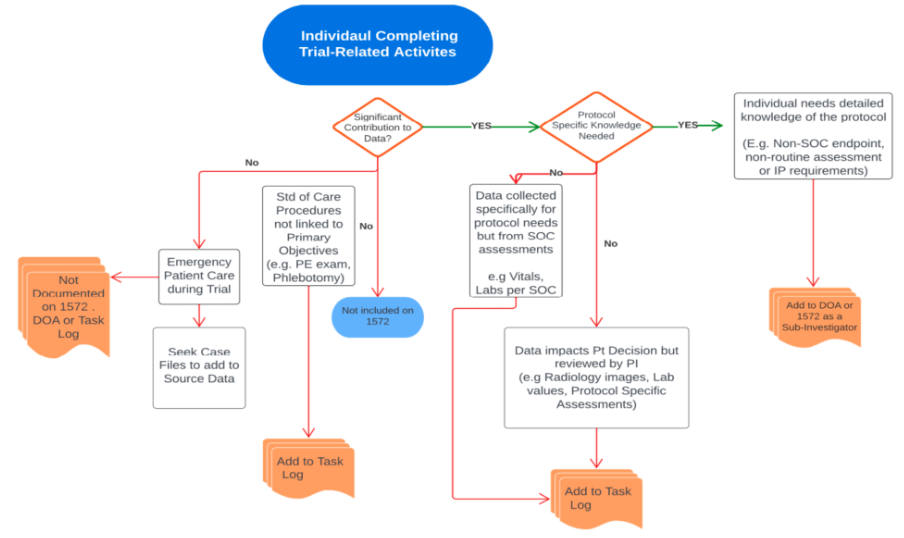
Decision elements to determine appropriate documentation of delegated trial-related activities