Bring Your Own Technology CoLab
Empowering Clinical Sites with the Tools They Trust
Summary:
BYOT enables clinical research sites to use their own validated technology in sponsor-led trials—improving efficiency, data quality, and patient experience.

The Problem:
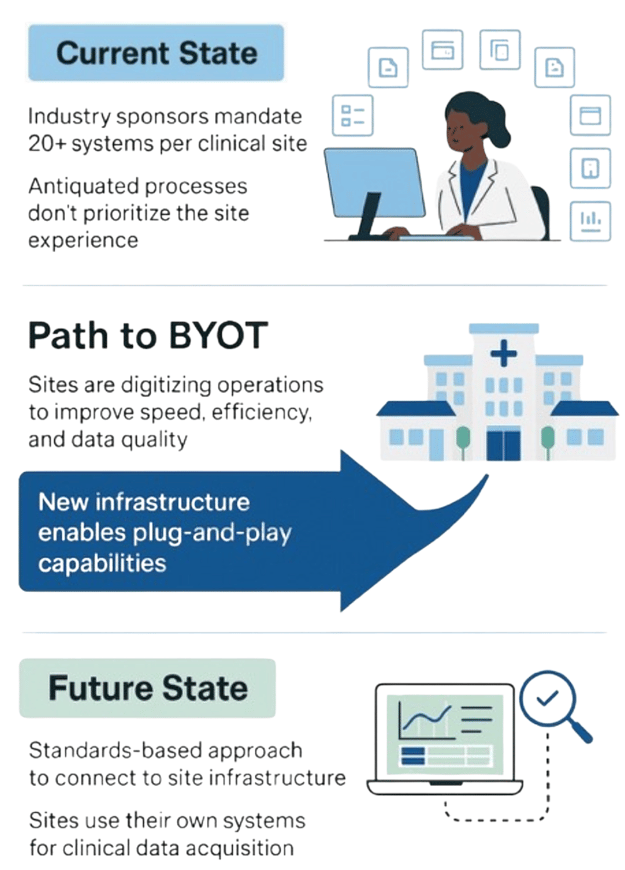
Current State of Clinical Site Technology
Clinical Sites Are Drowning in Sponsor Systems
- Sites are forced to use 20+ different systems for every sponsor trial.
- These systems are not integrated with how sites operate daily.
- Antiquated workflows and duplicated training waste time and increase risk.
- The site experience is rarely prioritized, despite their critical role in trials.
The Shift:
A New Paradigm is Emerging
Sites are taking back control
- Academic institutions and large hospital networks already use in-house tech.
- Independent sites and site networks are now investing in digital infrastructure.
- Goals: Speed, efficiency, better workflows, and higher data quality.
- The industry is standing at a tipping point—site-level digitization is scaling fast.
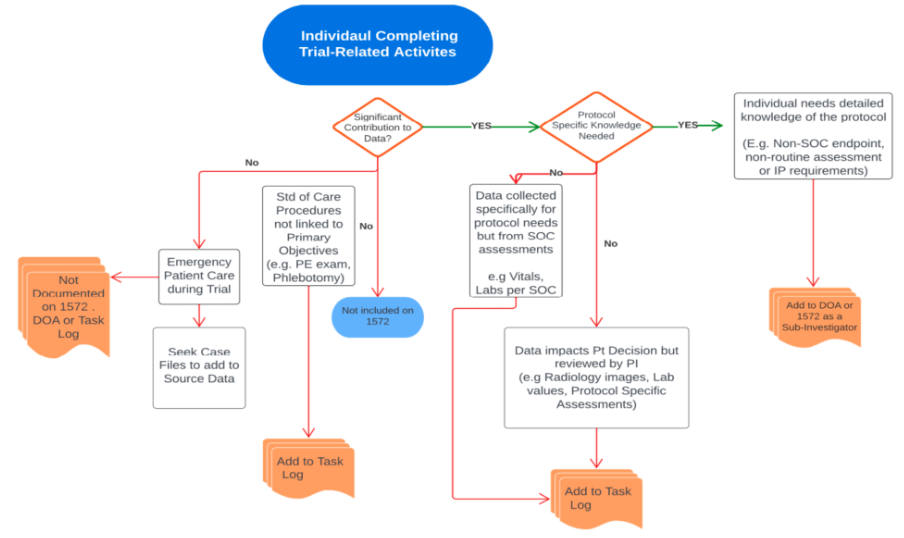
Decision elements to determine appropriate documentation of delegated trial-related activities
The Vision:
A Standards Based Future
A Connected, Flexible, Scalable Clinical Trial Ecosystem
- Sites use their validated platforms (eConsent, eSource, eISF)
- Sponsors gain faster, cleaner data via plug-and-play interoperability
- Based on compliance-ready protocols (21 CFR Part 11, ICH E6(R3), GDPR)
- Promotes fewer handoffs, lower cost, and shorter trial timelines
- Encourages vendor Trust Centers and shared Compliance Dossiers
- Maintains sponsor oversight and audit readiness
How BYOT Works
The BYOT Framework: Built for Flexibility and Rigor
- Horizon 1: Focus on eConsent (CURRENT)
- Site-led validation and sponsor approval
- No EDC integration required
- Horizon 2+: Expansion to eSource, eISF, DDC (FUTURE)
- Interoperable standards (CDISC ODM, USDM, FHIR)
- Scalable data ingestion models for sponsors
- A reusable documentation package that proves system validation and readiness Core Element: The Compliance Dossier
Who Benefits?
| Sites | Use familiar tools, reduce admin burden, improve morale |
| Sponsors | Get cleaner data faster, reduce protocol deviations, streamline oversight |
| Patients | Experience smoother visits, fewer tech frustrations, and better engagement |
| Vendors | Design once, reuse often—validated tools become “plug-and-play” |
Download the Playbook
Ready to learn more?
The full BYOT Playbook provides frameworks, validation guidance, implementation steps, and case studies to help your organization implement BYOT.
